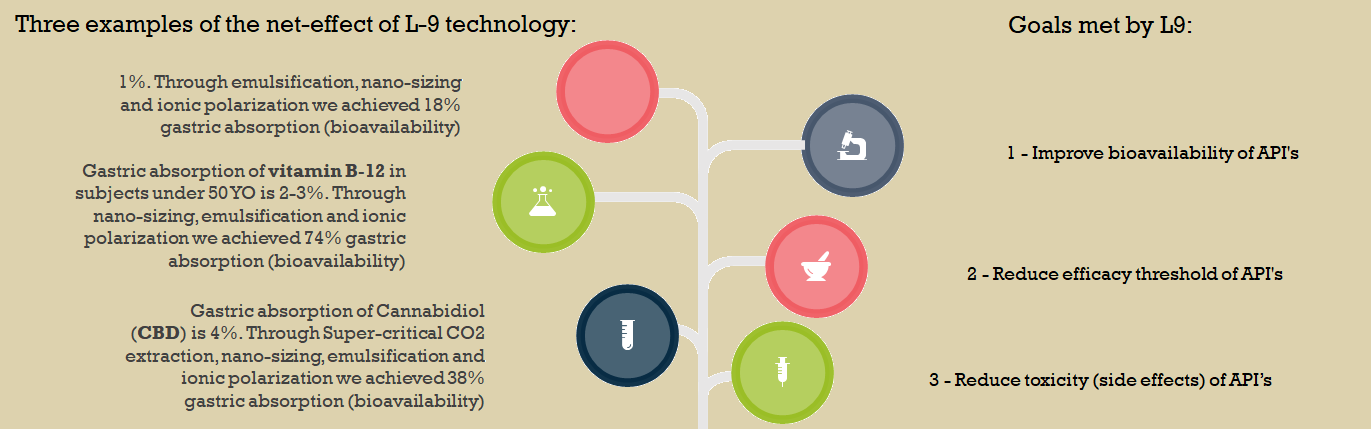
L9 BIOSCIENCE: OUR RESULTS
L9 BioScience’s prime market focus is the next wave in the pharmaceutical industry: | A Biosimilar is a biologic medical product highly similar to another already approved biological medicine. Biosimilars are approved according to the same standards of pharmaceutical quality, safety and efficacy that apply to all biological medicines. Biosimilars are officially approved versions of original "innovator" products. Reference to the innovator product is an integral component of the approval process as a new drug. This is different than a generic. A generic is a copy of the original innovator’s formula whose patent has expired. A Biosimilar is a new patented drug that is almost identical to a previously approved biological product, with no clinically meaningful differences in safety or efficacy. |
The PharmaCo landscape has changed!
New industry partnerships are being created globally to catch this wave. | For Ex.: Recently, a new partnership was created between the German Pharma, Stada (annual revenues of over $3.5 Billion cad) and Icelandic Pharma, Alvotech for the commercialization of 7 Biosimilars in all key markets. Their initial pipeline contains Biosimilar candidates aimed at treating autoimmunity, oncology and inflammatory conditions as well as ophthalmology for patients around the world. The originator products of the 7 Biosimilar targets currently generate over $50 billion in sales globally. |
L9 - Biosimilar Developed - Nortadalafil |
|

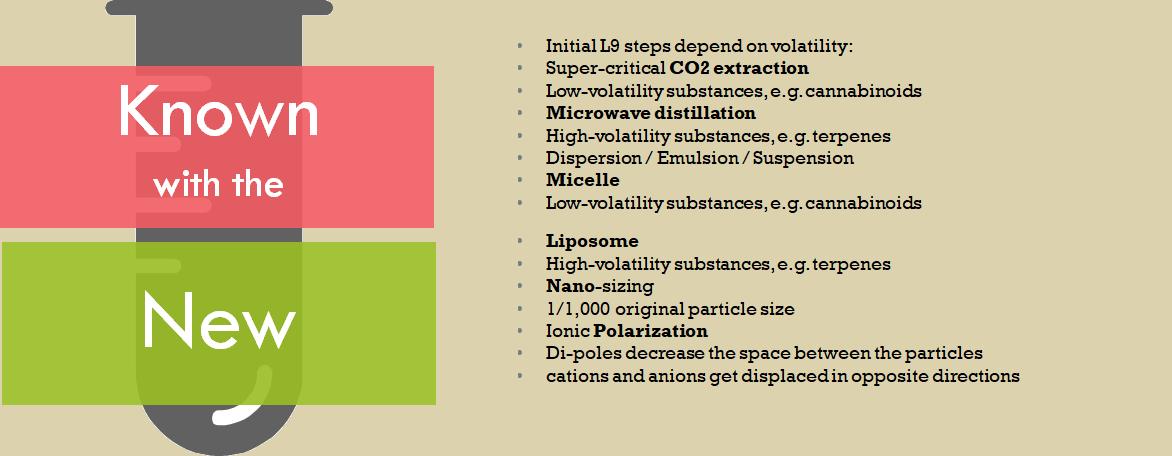

L9 production facility, we have developed working prototypes, a full-suite of IP including Patents, Trademarks and Copyrights.
- L9 BioScience:
- We make it Better – more effective
- We make it Cheaper – less API ingredient
- We create a new platform for Patent Development
- We provide a new path for previously failed drugs to succeed
Unique to the industry, L9 BioScience emerges as the wave of the future that is meeting the call from the consumer to discover wellness in a form that has no equal......
Other L9 BioScience Medical Development: P-53
It has been noted that many of the patients with cancer do not have the P53 gene. We have developed a formulation that stimulates the body to produce the P53 gene thereby greatly reducing the likelihood of developing cancer.
The P53 gene provides instructions for making a protein called tumor protein p53. This protein acts as a tumor suppressor, which means that it regulates cell division by keeping cells from growing and dividing (proliferating) too fast or in an uncontrolled way. The tumor suppressor gene P53 is mutated in ~50% of human cancers.
In addition to its function in tumor suppression, p53 also plays a major role in the response of malignant as well as non-transformed cells to many anticancer therapeutics, particularly those that cause DNA damage


